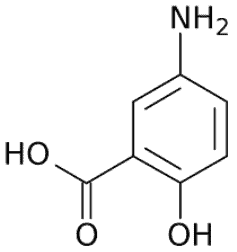
Mesalamine (Mesalazine)
Therapeutic Category
- Anti-inflammatory
Innovator Brand
GMP
DMF
Filed

Under Filing
Sample
Indication
Mesalazine is indicated for the induction of remission in patients with active or mild to moderate acute exacerbations of ulcerative colitis and for the maintenance of remission of ulcerative colitis. Prescribing information for mesalazine in the uk also indicates the medication for the maintenance of remission of crohn’s ileo-colitis.
CTX Lifesciences, is one of the leading Active Pharmaceutical Ingredients ( API ) manufacturer and supplier globally for Mesalamine (Mesalazine) with CAS Number: 89-57-6. We are a leading exporter of Mesalamine (Mesalazine) with CAS Number: 89-57-6. We are a trusted supplier with API exports in more than 87 Countries including the US, Europe, Brazil, Latin America, China, Korea,Iran, Middle East and other emerging markets.
CTX Lifesciences is one of the leading API manufacturer & supplier of Mesalamine (Mesalazine) with CAS Number: 89-57-6. This product is provided from our GMP compliant plant with product made under complete cGMP conditions. We provided with documentation support Drug Master File ( DMF ). Our manufacturing plant at Surat has accreditation by US-FDA, EDQM, EMA, ANVISA, KFDA, MOH-IRAN, Health Canada & Russian Authorities.
Disclaimer: CTX Lifesciences respects patent laws and conventions of pharmaceuticals as applicable in different countries.
API/Substances covered by patent are not offered to the countries where the patent law is in force. However, the final responsibility lies with the customer.